100% Silicone Foley Catheters
Excellence in design for reliable and comfortable acute and long-term urinary drainage in hospital or home care settings

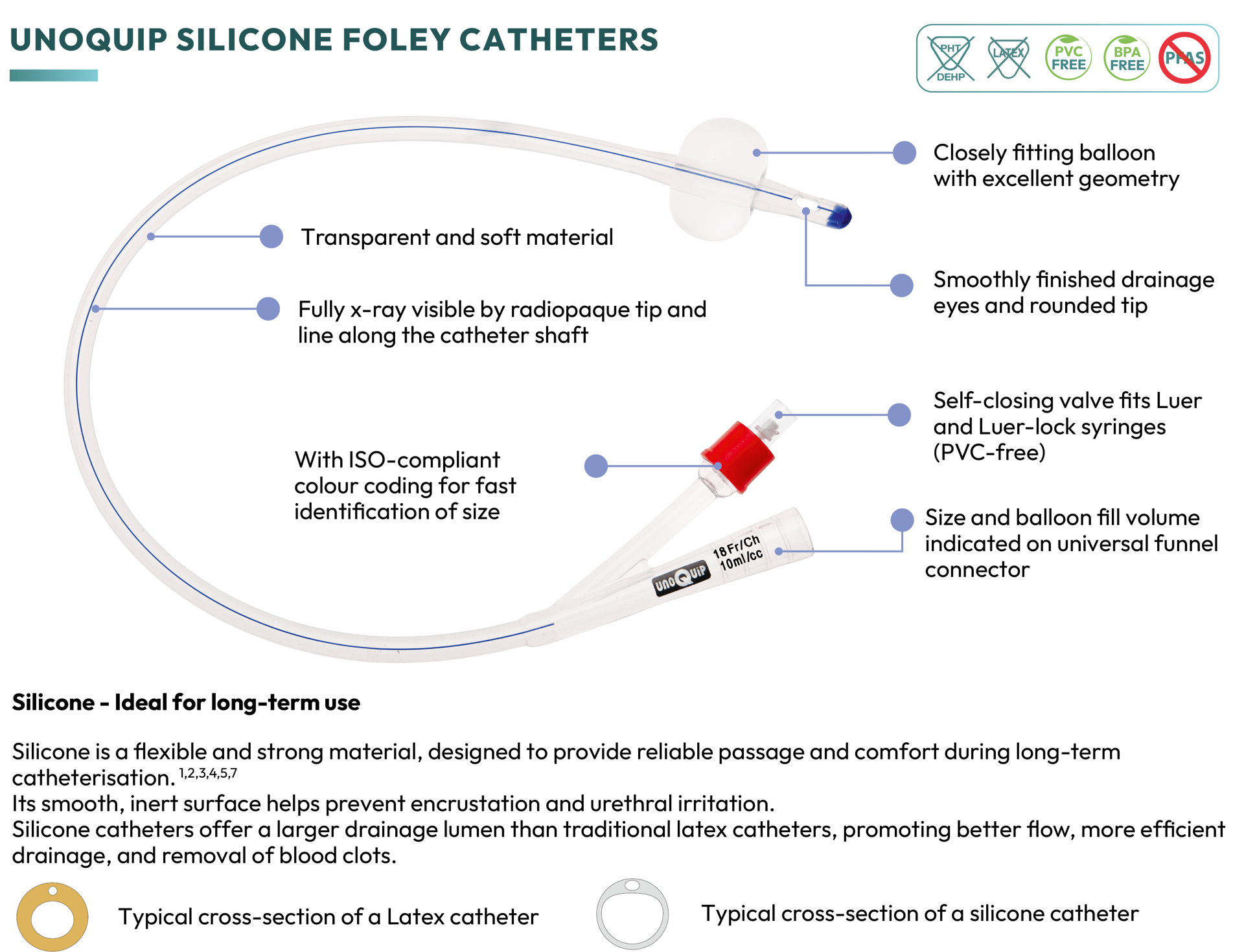
unoquip silicone foley catheters
- Silicone material is designed to provide reliable passage while facilitating comfort during long-term catheterisation.1,2,3,4,5,7
- UNOQUIP 2-way silicone Foley catheters can be retained in the human body for up to 90 days.
- Comprehensive range including male, female, paediatric, open tip, Tiemann tip, temperature-sensing, and postoperative catheters
- UNOQUIP silicone Foley catheters are entirely PVC-free (PVC material may still be utilised by other brands for the valve).
- 2-way catheters (sizes 12 to 18) can also be used for replacement of suprapubic catheters (exchange only).
Long-term usage up to 90 days after insertion
UNOQUIP 2-way silicone Foley catheters can be retained in the human body for up to 90 days. The use of sterile aqueous glycerine solution enhances long-term use by significantly reducing diffusion through the catheter balloon, thereby minimising loss of fill volume.
All UNOQUIP 2-way Foley catheters are optionally available as kits, including a syringe prefilled with 10% sterile aqueous glycerine solution. Additionally, the kits contain an empty syringe to support catheter replacement.

Without glycerine
While water easily diffuses through silicone balloon membranes, causing over 50% volume loss during long-term catheterisation, glycerine solution remains stable due to minimal diffusion.5,6,7,8,9
With glycerine
- Catheter is securely anchored, minimising dislodgement risk
- No regular checks and topping up required
- Time and cost savings due to reduced maintenance
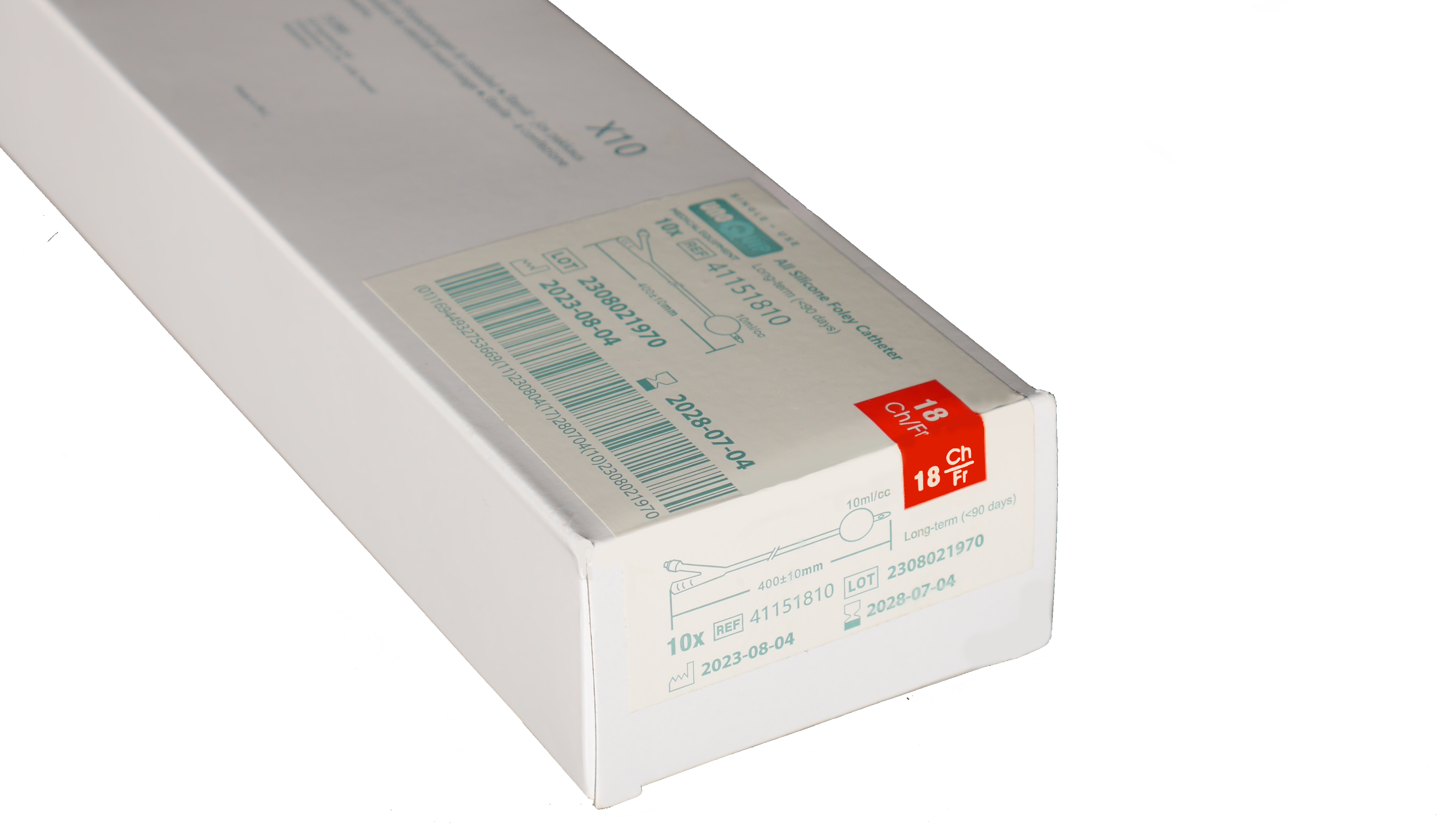
Easy-to-Read Labels for Quick Identification and Access
All shelf box labels display the catheter type and size including ISO colour coding for quick catheter and size identification.
The wrap-around labels ensure visibility from the top and on the front flap of the shelf box.
UNOQUIP Silicone Foley Catheter Options for Hospital and Home Care
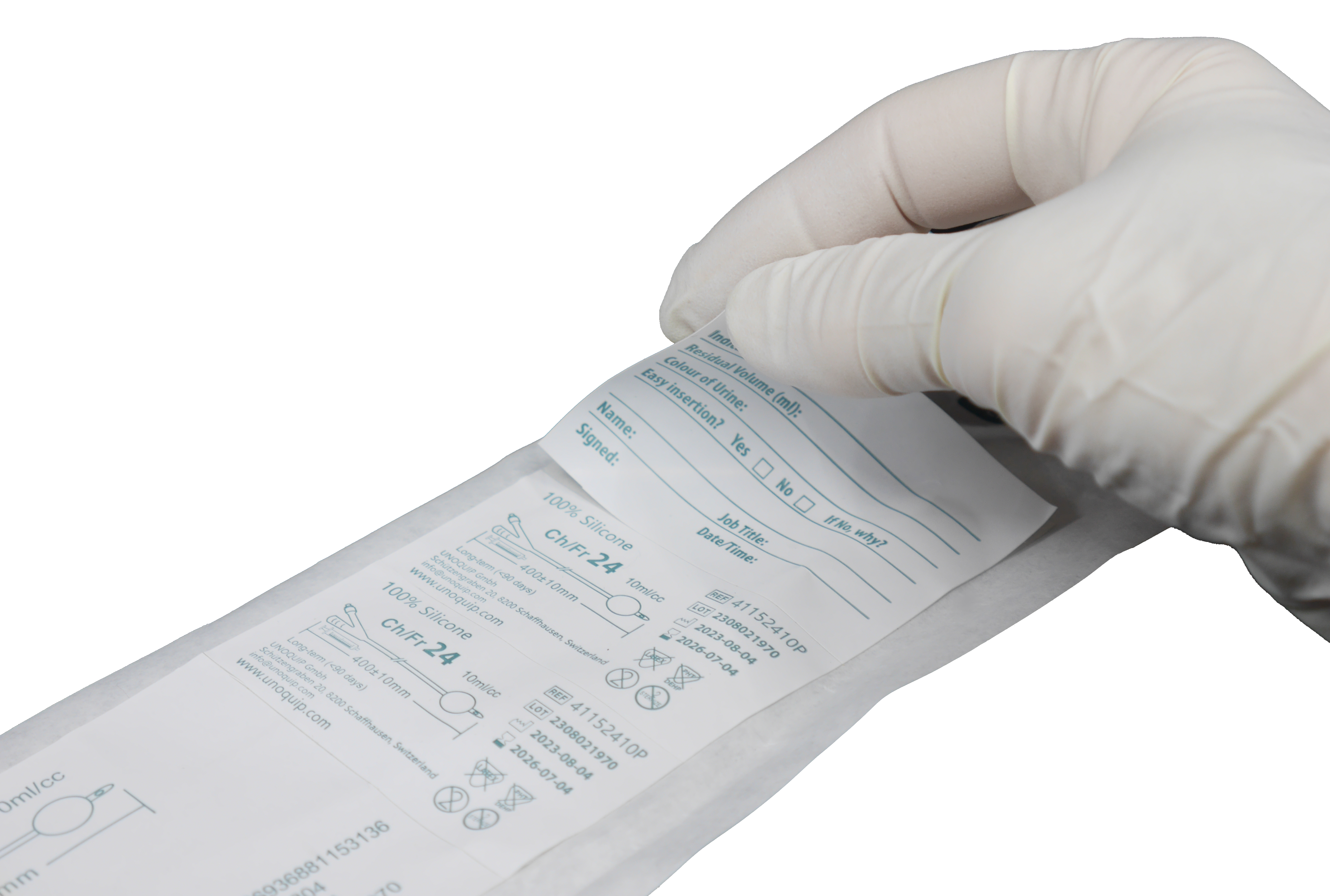
Peel-off labels with catheter details, including reference code and batch number, for full traceability, and fields to document key catheterisation details.
Please see our complete range and ordering information here.
References:1 Nacey JN, Tulloch AGS, Ferguson AF. Catheter-induced Urethritis: A Comparison Between Latex and Silicone Catheters in a Prospective Clinical Trial. British Journal of Urology 1985;57:325-328; 2 HUANG et al. Effect of silicon and latex urinary catheters: a comparative study, 2005; 3 Tunney MM, Keane PF, Gorman SP. Assessment of urinary tract biomaterial encrustation using a modified Robbins device continuous flow model. J Biomed Mater Res. 1997;38(2):8; 4 Morris NS, Stickler DJ, Winters C. Which indwelling urethral catheters resist encrustation by Proteus mirabilis biofilms? British Journal of Urology 1997;80,58–6; 5 Evidence-based Guidelines for Best Practice in Urological Health Care Catheterisation - Indwelling catheters in adults. Urethral and Suprapubic, European Association of Urology Nurses 2024. 6 Studer UE, Bishop MC, Zingg EJ. How to fill silicone catheter balloon. Urology. 1983 Sep;22(3):300-2; 7 JoAnn Mercer Smith, BSN, RN, CWOCN, Indwelling Catheter Management: From Habit-based to Evidence-based Practice, Ostomy Wound Manage. 2003;49(12):34-45; 8 Long-term indwelling silicone foley catheter test report. 2015. In vitro test report. Copy on file; 9 Test report for 10% glycerin solution. In vitro test report. Copy on file.
